In the future, storing solid state hydrogen will be more and more important for our independence. Besides the global warming effect caused by using fossil resources, they are finite. When there will be no more fossil fuels available, humanity needs to look for an alternative way of energy usage.
by Niclas Jan Miers, Thanks buddy,
the entire article is only readable for members.
Executive Summary
In nowadays, modern world, the usage of hydrogen either as energy carrier, or for chemical processes becomes more and more interesting. Due to that, production, and consumption methods, but especially storing methods for automotive applications need to be further developed. For this, modern so-called solid-state hydrogen storages are developed which use nanopowders to store hydrogen in a chemical bonding. This thesis is based on such nanopowders, produced in a previous internship at the Helmholtz-Zentrum Geesthacht (HZG) [1]. Requiring light storage weights for automotive applications, the nanopowders are based on light metal hydrides. They are composed of different quantities of Magnesium, Lithium, Nitrogen, Boron and Hydrogen. In this thesis, parameters, such as apparent density, thermal conductivity, hydrogen storage capacities, as well as optimum temperature and pressure conditions for the hydrides are measured and evaluated. The aim of this thesis is to determine the performance of the powders in temperature range between 1200 C and 190 0 C With pressure conditions between 1 bar and 75 bar. Predictions on how much hydrogen can be stored in a specific volume are made by combining the determined densities With measured hydrogen storage capacities. Finally, a perspective shall give an overview about the future of hydrogen storages and which important role the powders might Play.
1 Hydrogen as energy carrier
Water will be the coal of the future. Water, decomposed into its primitive elements by electricity. Hydrogen and oxygen, which constitute it, used singly or together, will furnish an inexhaustible source of heat and light, of an intensity of which coal is not capable. Someday the coal rooms of steamers and the tenders of locomotives will, instead of coal, be stored with these two condensed gases’
Jules Verne (Opus: The mysterious island, 1874/75)
Jules-Gabriel Verne, born on the 18th of February 1828 in Nantes and died on the 24th of March 1905 in Amiens, was a French novelist. He was a pioneer of the late 19 th century and founded, besides Hugo Greenback, Kurd Laßwitz and H. G. Wells, the science fiction literature. When publishing his novel ‘The mysterious island’ in 1874/75, he already knew about the high potential reaction between hydrogen and oxygen, which results only in water. During the late 18th century, a tremendous, worldwide remodelling of working conditions started and the use of steam machines and fossil energy sources revolutionized work. In the late 19th century, humans began to assume, that the release of greenhouse gases might affect the climate on earth. Though, calculations based on this theory were strongly doubted until 1960, when more and more scientists started to believe the theory that carbon dioxide causes a warming effect [2]. The idea of using hydrogen as an energy carrier already existed at that time and people began to think about alternatives at the latest after the energy crisis in 1974 [3]. Since the 1990’s advanced computer simulation led to a better understanding of climatic processes and changes like earlier glacial periods and the important influence of greenhouse gases on global warming. Regular UN-CLIMATECONFERENCES were established and various treaties and agreements were signed on government level with the target of limiting C02 output and global warming [4]. The two biggest problems of the climate change are still the use of fossil energy carriers for power generation (electricity, heating, cooling) and the use of fossil fuel in the automotive sector. Nowadays it is more important than ever before to minimize or better avoid whenever possible the use of ‘grey’ energies in industrial production and private consumption as well. What if fossil energy would not be needed anymore? This might not happen in the near future, but with the rapid expansion and use of alternative energies (Figure 20, Figure 21 and Figure 22), humanity is on a good way to improve the climate.
Due to the rising quantity of renewable energies, power storages and buffers in the supply grid are indispensable. These buffers are used for storing energy, when renewable power plants produce more power than demanded. In opposite, if there is more energy demand than produced, these buffers release the stored energy. While the technological development of power storages is at a level, where power can be stored in large scale storages such as pumped hydroelectric energy storages (PHES), big battery farms or compressed air energy storages (CAES) in stationary plants, great progress needs to be made when it comes to small energy storages for automotive
applications. At present, two major principles exist to store energy in electric vehicles. Either vehicles are equipped with a large battery pack, or with a hydrogen-bay system. In comparison to battery-based systems, hydrogen-based systems to have a heavy battery, but include hydrogen tanks, a fuel cell and a small batter:
smoothen power peaks. While modern Li-lon batteries have a maximum er—— density of 0,15-0,18 kWh/kg [5], hydrogen in its elementary form can s-z-; 33,33 kWh/kg. So, to store the energy of 1 kg hydrogen in a battery, an amour:
approximately 200 kg (Li-lon) and 889 kg (Lead-Acid) [5] is needed. In addition to their high weight, conventional batteries also have disadvantages regarding to t-echarging time and non-stable cycling behaviors.
The automotive industry is facing new challenges by developing greenhouse gas fee vehicle drives. In the next chapter, principles and ideas on use and storage of hydrogen are explained in more detail.
1.1 Applications for Hydrogen
The usage of hydrogen can help to assure human life standards when fossil sources run short. Nowadays its outstanding properties and features make it already a high potential energy source for lots of applications. That includes heating, cooling arc power supply for households as well as mobility applications. It can be used i” consumers such as internal combustion engines, catalytic burners, heaters and fuel cells. Although the oxidation of hydrogen is exothermal, it is differentiated between hc: and cold combustion [6]. The hot combustion of hydrogen describes its burning oxidation with a flame (conventional combustion engines or catalytic burners). Colc combustion mainly refers to the electrochemical net-reaction in fuel cells. Both have different reaction steps, but the reaction path 2 1-12 + 02 + 2 H20 is the same.
Parts such as turbines and combustion engines are exposed to mechanical stress. whereas fuel cells run without friction losses. This does not only require less maintenance, also the life span and efficiency is much higher. In opposite to the hot combustion where hydrogen is first mixed with oxygen and then burned, fuel cells consist of electrodes, which are separated by an electrolyte membrane. At the anode hydrogen is divided according to the reaction H2 2H+ + 2e-. At the cathode, oxygen is added and the reaction % 02 + 2e- + 2H+ —5 H20 occurs [6, 7]. Unlike the hydrogen ions, electrons cannot pass through the electrolyte, which makes them take a detour around the electrolyte membrane. This causes a current to run electrical peripherals.
Controlling the high reactive reaction of hydrogen and oxygen in a fuel cell, makes hydrogen an interesting fuel for mobile applications. Vehicles such as forklifts, buses and trains already run on hydrogen, while cars are still in developing state. This mainly refers to the smaller space available for tanks in the car in conflict with longer driving distances. The next chapter refers to the existing storage technologies for stationary and mobile applications.
1.2 Hydrogen storage
As long as fuel cells are operated in an oxygen-containing atmosphere, oxygen for the reaction can be taken from the surrounding air, while hydrogen needs to be stored in tank systems (Figure 1). The very high mass related, but very low volume related energy density of hydrogen, requires storage tanks capable to store as much hydrogen per mass as possible in a small volume. State of the art for mobile applications is to store hydrogen in high-pressure tanks suitable for 300 bar (mainly for buses) or 700 bar tanks (mainly for cars), respectively. These physical-based tanks have a stable cycling behaviour of 100 %, which makes them very interesting for systems where many charging cycles are expected. High-pressure tanks store hydrogen at a 227 times (300 bar), or even 434 times (700 bar) higher density then in its natural form. To reach such high pressures, up to 12 % of the stored energy is needed for compression [8]. Further methods of physical-based hydrogen storages are liquid H2 and cold/cryo compressed systems. Liquid 1-12 systems store hydrogen at its melting point of -252,8 o c. However, up to 46 % of the storable energy is already needed to liquefy the hydrogen [8].
How is hydrogen stored?
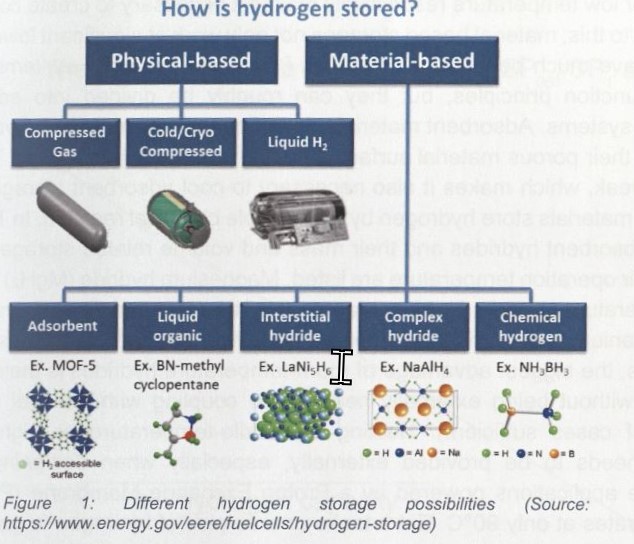
Ex. MOF-5 Ex. BN-methyl cyclopentane
O = H2 accessible
Figure 1: Different hydrogen storage possibilities (Source: https://www.energy.gov/eere/fuelcells/hydrogen-storage)
Combined with the losses of hydrogen due to overpressure release, this hydrogen storage method has a very low efficiency. Cold/cryo compressed tanks are hybrid systems of high pressure and cryogenic H2 storages. They store hydrogen at lower temperatures than liquid H2 storages, combined with high pressures. The hydrogen density of up to 67,5 kg H2/m 3 is very good when ignoring the effort for insulation and high-pressure construction [9]. Fluid 1-42 and cold/cryo compressed tanks are not 100 % adiabatic. Consequently, blowing off hydrogen is necessary to release overpressure.
Table 1: Different hydrogen storages with storage capacity and operation temperature (based on [6, 9 to 1 Z.
| Storage form | H2 capacit | operation temperature [° C] | ||
| kg H2/m 3 | wt.% | |||
| Compressed H2 300 bar | 17 | 100 | -80 | |
| Compressed H2 700 bar | 33 | 100 | -80 | |
| Fluid H2 | 71 | 100 | -252,8 | |
| Cryo compressed | 67 | 100 | -220 | |
| Hydride | M H2 | 54 | 300 | |
| 42 | 125-180 | |||
| FeTiH2 | 102 | room temperature | ||
Neglecting the storage vessel, physical based hydrogen storages have 100 wt. % of h2 capacity (Table 1), but the problem with the high fluctuation of hydrogen and the high pressure or low temperature respectively makes it necessary to create complex tanks In contrast to this, material based storages not only work at significant lower pressures but also have much better safety features [13]. Material based systems have man: different function principles, but they can roughly be divided into adsorbent anc absorbent systems. Adsorbent materials do not chemically react with hydrogen. They store it on their porous material surface by using van der Waals forces. These forces are very weak, which makes it also necessary to cool adsorbent storages. Contrary. absorbent materials store hydrogen by a reversible chemical reaction. In Table 1 , three different absorbent hydrides and their mass and volume related storage capacity, as well as their operation temperature are listed. Magnesium hydride (MgH2) is a so-called high-temperature hydride, Natrium alanate (NaAlH4) is a middle-temperature hydride and Iron titanium (FeTiH2) is a room-temperature hydride. Comparing these three types of hydrides, the biggest advantage of room-temperature hydrides is their capability of operation without being externally heated (the coupling with the fuel cell is in the majority of cases sufficient). Heating of middle-temperature or high-temperature hydrides needs to be provided externally, especially when designing a tank for automotive applications powered by a Proton Exchange Membrane (PEM) fuel cell which operates at only 90 0 C. Extra tanks or a surplus of hydrogen in gaseous phase (hybrid system) must be carried to ensure a hydrogen supply in the cold phase of the main storage. Absorptions in these hydrides either occur in an easy reaction, where molecular hydrogen splits into atoms and then connects to a crystalline surface structure (FeTiH2), or it binds to reaction partners in a mainly covalent bonding (NaAlH4, MgH2). The hydrogenation and dehydrogenation mechanism of metal hydrides can also be used to purify hydrogen. According to the work of Wenzel et al., FeTi-based systems can purify hydrogen from 99,9 % to 99,9999% purity by dumping the first 10 % of released hydrogen [6, 14]. These first 10 % of hydrogen then have a very high quantity of CH4, N2, 02, CO and C02. Referring to Table 1, a room
2 Scope of work
At the Helmholtz-Zentrum Geesthacht in the Institute of Materials Research, nanopowders are developed to be used for hydrogen storage in different application fields inter alia in the automotive sector. These powders can become essential as a hydrogen storage for fuel cell powered cars. Due to the new development of storage powders such as SP05-SP4, very few data exist to specify their characteristics. To be able to use these hydrogen storage powders in cars, characteristics such as density, thermal conductivity and especially their kinetic behaviours for absorption and desorption at different temperatures and pressures are essential. It also influences the factor for storable hydrogen quantities in a given time. Especially thermal conductivity, density and kinetic characteristics are important to know for subsequent tank simulations [17].
SP05, SPI and SP4 have the same starting material, but their different LiBH4 amounts, should lead in theory to different storage capacities (Figure 2). Also, referring to [1 6], they have differences in their kinetic behaviour due to different thermodynamic properties. In this work, the parameters for thermal conductivity, as well as their apparent density and their kinetic characteristics are measured and evaluated. A conclusion based on the measured data is given, as well as a perspective on which further steps with the powders are possible.