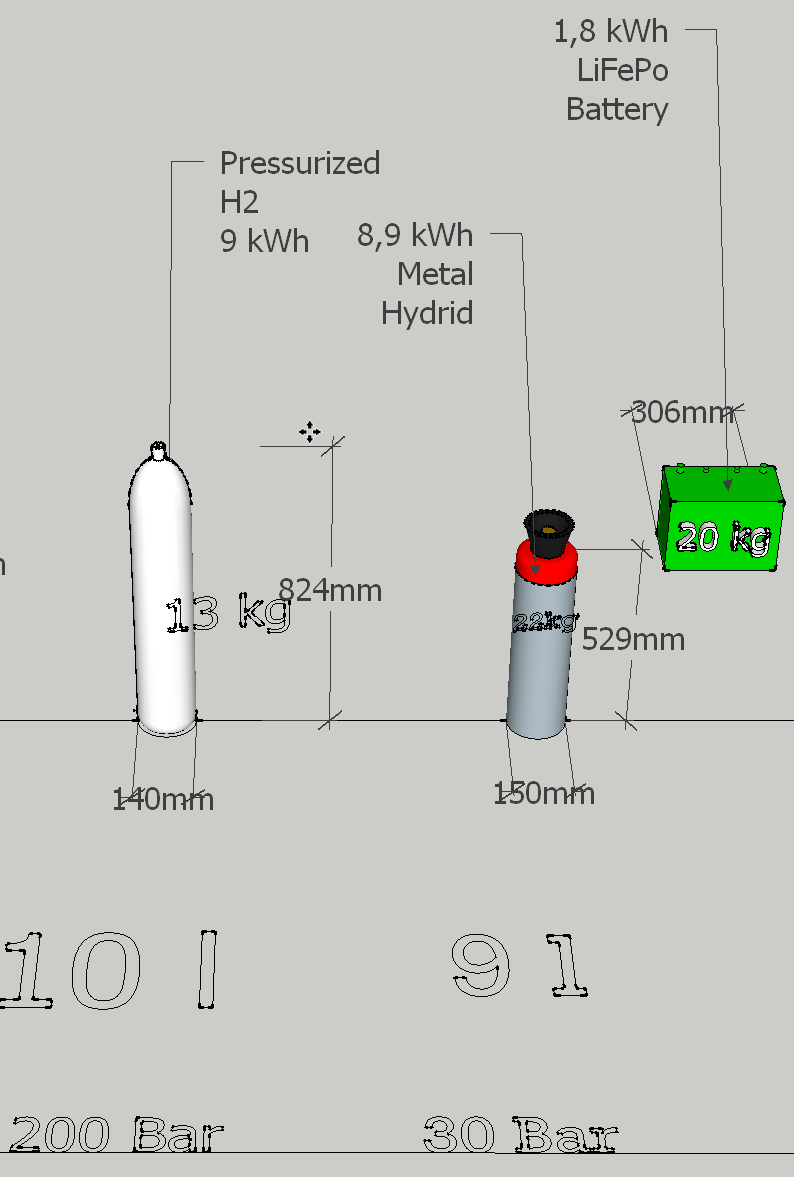
Metal hydride hydrogen storage cylinders can hold much more hydrogen than other hydrogen storage possibilities like high pressure steel cylinders. The metal hydride canisters for hydrogen are small in size and portable, thus perfect for mobile applications.
We are so excited to have the first price for a market ready metal hydride hydrogen storage. But the prices for hydrogen storage solutions are only one part of the equation.
Durability, contaminants, recyclability and of course the energy density are important factors for metal hydride hydrogen storages.
The price of metal hydride cylinder is the biggest drawback
This phenomena that solid metal can bind hydrogen atoms is known very long. Every once in a while a startup recovers the hope in an energy dense hydrogen storage. Sadly they haven’t gotten widely used in the hydrogen economy, even though they are available on the market for decades.

The first metal hydride storage cylinder manufacturer answered our questions.
They offer for example a hydrogen cartridge with the equivalent of 3000 liters of hydrogen stored in a bottle only 2 feet long with a diameter of 15 cm.
- What Metals are used in metal hydride hydrogen batteries?
- What is the Charge and discharge temperature of the metal hydride hydrogen storage?
- What are the losses between charge and discharge?
- What is the time for charging?
- What is the charging pressure for metal hydride bottles?
- Is there any contamination of the hydrogen through the storage?
- What happens with water accumulation in the cylinder when temperature drops?
- How can metal hydride cartridges be regulated?
- What is the discharge pressure of a metal hydride canister?
- Is the discharge of metal hydride canisters linear?
- Is there any degradation?
- How many cycles do you estimate for the best hydrogen storage solution in metal hydrides?
- What is the thread on the bottleneck or the metal hydride bottle valve?
- Conclusion: Are metal hydrides economically suggestable and sustainable?
- Hybrid Storage
- metallic hydrides
What Metals are used in metal hydride hydrogen batteries?
The experts answer was:
2 thoughts on “Price for Metal Hydride Hydrogen Storage?”
Comments are closed.